Enantioselective α-amination of 1,3-dicarbonyl compounds in batch and flow with immobilized thiourea organocatalysts†
Abstract
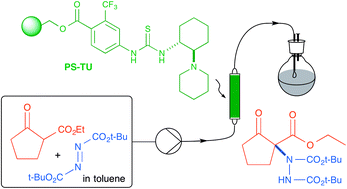
A polymer-supported bifunctional thiourea organocatalyst (PS-TU) has been prepared and successfully used in the enantioselective α-amination of 1,3-dicarbonyl compounds with azodicarboxylates. In contrast to homogeneous thioureas, PS-TU is not irreversibly deactivated by the azodicarboxylate reagents, and simple washing with triethylamine between runs has allowed the reuse (9 cycles) of the PS-TU catalyst. The α-amination mediated by PS-TU has also been adapted to perform the enantioselective amination (93% ee) of ethyl 2-oxocyclopentanecarboxylate in continuous flow (7.5 h operation, 21 min residence time, TON = 37).

 Please wait while we load your content...
Please wait while we load your content...