Chiral ureas and thioureas supported on polystyrene for enantioselective aza-Henry reactions under solvent-free conditions†
Abstract
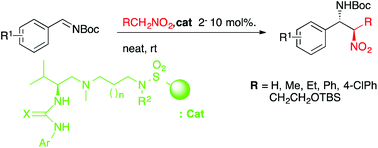
Novel bifunctional ureas and thioureas immobilized on sulfonylpolystyrene have been prepared as recoverable and reusable organocatalysts and have been used in the stereoselective aza-Henry reaction under solvent-free conditions. The activity and stereoselection of the catalysts are dependent on the length of the tether bridging the active site and the polymer, the catalyst derived from 1,6-hexane diamine being the best one. It has also been demonstrated that the supported catalysts are more effective than the homologous soluble catalysts.
- This article is part of the themed collection: Elemental Recovery and Sustainability

 Please wait while we load your content...
Please wait while we load your content...