Fluoride-free synthesis of a Sn-BEA catalyst by dry gel conversion†
Abstract
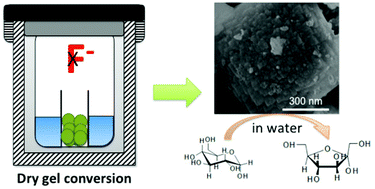
Sn containing molecular sieves with BEA topology (Sn-BEA) are active Lewis catalysts for a large variety of redox reactions. However, the synthesis of Sn-BEA often requires the use of toxic chemicals (e.g. hydrofluoric acid). In this study, we demonstrated that an active Sn-BEA catalyst can be directly synthesized in a non-fluoride medium via a dry gel conversion method with the aid of seed crystals. It was shown that seeding of zeolite BEA crystals can facilitate crystal growth and lead to BEA topology with framework Sn. Furthermore, it was found that ion-exchange with ammonium ions is indispensable to retain the crystal structure during calcination. The catalytic activity of the Sn-BEA catalyst was evaluated by the isomerization of glucose and reaction of pyruvaldehyde in the aqueous phase, and compared with the conventional hydrophobic Sn-BEA synthesized in a fluoride medium. The catalytic activity of the Sn-BEA synthesized in the absence of fluoride was found to be lower than that of the conventional Sn-BEA for catalyzing the isomerization of glucose in the aqueous phase, which could be attributed to its hydrophilic surface. For the reaction of pyruvaldehyde in the aqueous phase, the Sn-BEA synthesized in this study showed similar activity to the conventional hydrophobic Sn-BEA, indicating that the catalytic activity of Sn-BEA catalysts largely depends on the type of reactions. The current work provides the first example of direct preparation of a Sn-BEA catalyst in a caustic medium.

 Please wait while we load your content...
Please wait while we load your content...