A strategy to overcome the thermodynamic limitation in CO2 conversion using ionic liquids and urea
Abstract
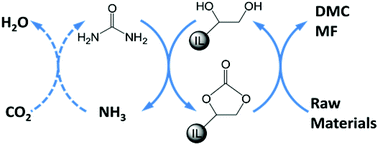
Enhancing the equilibrium conversion of thermodynamically unfavorable reactions is a very interesting and challenging topic for chemists. The unique properties of ionic liquids (ILs) provide new opportunities to solve this problem. Herein a strategy is proposed to circumvent the thermodynamic limitation of chemical reactions using the designable and non-volatile nature of ILs. In this approach a reactant first reacts with a functional IL to form a high energy IL intermediate, which can further react with other reactants to yield products and regenerate the IL. To verify the feasibility of this strategy, the syntheses of dimethyl carbonate (DMC) and methyl formate (MF) via urea, which are very important reactions used to convert CO2 but are thermodynamically unfavorable, were conducted with the aid of a diol IL. It was found that the equilibrium yields of DMC and MF obtained by this methodology could be 64 times and 9 times higher, respectively, than those of the conventional methods.

 Please wait while we load your content...
Please wait while we load your content...