Mixed NaNbxTa1−xO3 perovskites as photocatalysts for H2 production†
Abstract
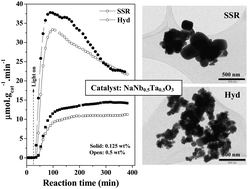
Mixed perovskites NaNbxTa1−xO3 were prepared by solid state reaction (SSR) as well as by hydrothermal (Hyd) methods, and their photocatalytic activity for hydrogen production was studied using the water–methanol system. The assessment of the NaNbxTa1−xO3 materials obtained by the SSR method reveals that the activity of the individual NaTaO3 and NaNbO3 perovskite semiconductors is largely improved in their combined form. Among several compositions employed, the 1 : 1 molar ratio (NaNb0.5Ta0.5O3 sample) shows the best performance for H2 production. On the other hand, using the Hyd method, which implies lower synthesis temperature, the photocatalytic activity of NaNb0.5Ta0.5O3 is enhanced compared to the material obtained by the high temperature SSR method. The characterization of the materials reveals that catalyst properties like high surface area, a larger proportion of the monoclinic crystalline phase and lower crystal defects for the NaNb0.5Ta0.5O3 photocatalyst synthesized by the hydrothermal route may be responsible for its superior activity. Further significant improvement in the activity of the NaNb0.5Ta0.5O3 semiconductor is achieved by the addition of Pt as the co-catalyst, showing that the loading amount has a great influence on the activity. The highest H2 production rate (37.8 μmol g−1 min−1) is obtained for the catalyst prepared by the hydrothermal method (Hyd-NaNb0.5Ta0.5O3) with 0.125 wt% of Pt loading. Moreover, the developed Hyd-NaNb0.5Ta0.5O3 sample shows a stable H2 evolution activity for several reuse cycles.

 Please wait while we load your content...
Please wait while we load your content...