Zemplén transesterification: a name reaction that has misled us for 90 years†
Abstract
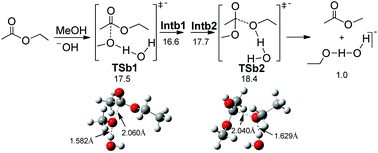
We demonstrated that using NaOH and NaOMe in methanol for deacylation are identical, indicating that the Zemplén condition has been misleading us for almost 90 years. The traditional base-catalyzed mechanism cannot be used to explain our results. We propose that H-bond complexes play key roles in the base-catalyzed process, explaining why deacylation in methanol can be catalyzed by hydroxide.

 Please wait while we load your content...
Please wait while we load your content...