Syntheses of novel halogen-free Brønsted–Lewis acidic ionic liquid catalysts and their applications for synthesis of methyl caprylate
Abstract
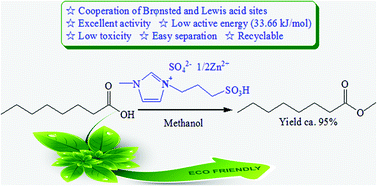
A series of benign halogen-free ionic liquid (IL) catalysts were synthesized by combining the Brønsted acidic ionic liquid [HSO3-pmim]+HSO4− with ZnO in different composition ratios. The IL catalysts, which possess both Brønsted and Lewis acidities, were employed as acidic catalysts for the esterification of n-caprylic acid to methyl caprylate. The [HSO3-pmim]+(1/2Zn2+)SO42−, prepared by cooperating equimolar amounts of Brønsted and Lewis acid sites, was found to exhibit an optimal catalytic performance and excellent durability. This is attributed to a synergy of Brønsted and Lewis acidities manifested by the catalyst. The response surface methodology (RSM) based on the Box–Behnken design (BBD) was utilized to explore the effects of different experimental variables (viz. catalyst amount, methanol to caprylic acid molar ratio, temperature, and reaction time) on the esterification reaction. Analysis of variance (ANOVA) was also employed to study the interactions between variables and their effects on the catalytic process. Accordingly, the deduced optimal reaction conditions led to a high methyl caprylate yield of 95.4%, in good agreement with experimental results and those predicted by the BBD model. Moreover, a kinetic study performed under optimal reaction conditions revealed an apparent reaction order of 1.70 and an active energy of 33.66 kJ mol−1.


 Please wait while we load your content...
Please wait while we load your content...