Quantitative synthesis of bis(cyclic carbonate)s by iron catalyst for non-isocyanate polyurethane synthesis†
Abstract
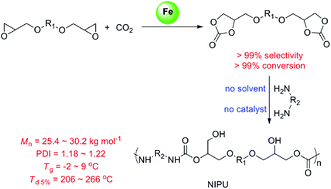
Bis(cyclic carbonate)s were quantitatively prepared with high efficiency via the coupling reaction of carbon dioxide (CO2) with diglycidyl ethers by a [Fe(BPMCDAC)]/TBAB catalytic system, where glycol diglycidyl ether (1a) could be completely converted to the corresponding bis(cyclic carbonate) (2a) with a turnover number of 1000 at 100 °C and 3 MPa in 4 h. The obtained bis(cyclic carbonate) (2a) could be used to prepare hydroxyl-functional polyurethanes via reaction with diamines, which may be one alternative for obtaining conventional polyurethanes without the use of toxic phosgene or isocyanates. The number-average molecular weights of the obtained non-isocyanate polyurethanes (NIPUs) were up to 25.4–30.2 kg mol−1, and the polydispersity indexes (PDIs) were relatively narrow between 1.18 and 1.22. A typical NIPU showed a glass transition temperature of 9 °C and an initial degradation temperature (Td 5%) of 206 °C.

 Please wait while we load your content...
Please wait while we load your content...