The α-chymotrypsin-catalyzed Povarov reaction: one-pot synthesis of tetrahydroquinoline derivatives†
Abstract
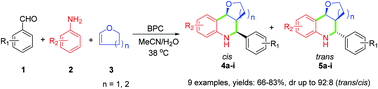
The three-component one-pot Povarov reaction for the synthesis of tetrahydroquinoline derivatives was catalyzed by α-chymotrypsin from bovine pancreas (BPC) for the first time. The products were obtained in moderate to good yields with a wide range of substrates. Based on the control and comparison experiments of natural and promiscuous activities, a tentative mechanism was discussed. Molecular docking and energy calculation of quantum chemistry were used to explain the experimental results in theory. As a novel case of enzyme catalytic promiscuity, this work expands the application of BPC. Exploring the untapped catalytic promiscuity of natural enzymes may also provide useful information about enzyme evolution.

 Please wait while we load your content...
Please wait while we load your content...