Etherification of glycerol with isobutene on sulfonated graphene: reaction and separation
Abstract
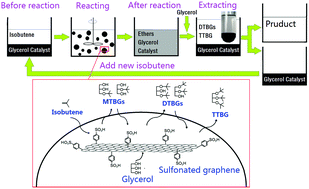
A sulfonated graphene catalyst (SG) prepared by grafting sulfonic acid-containing aryl radicals onto the two-dimensional surface of graphene was used for the etherification of glycerol with isobutene, and a reaction–extraction process was developed for easy realization of product isolation and catalyst recycling. With its ultra thin two-dimensional open substrate, stable sulfonic acid sites, amphiphilic properties and light texture, SG exhibited excellent catalytic performance in the etherification reaction. At 60–70 °C with 4 wt% catalyst loading and a molar ratio of isobutene/glycerol 4, a nearly complete conversion of glycerol in 7 h and a selectivity of more than 90 mol% to desired multi-butyl glycerol ethers were achieved. Moreover, undesired oligomerization of isobutene was successfully suppressed. When extracted with fresh glycerol, the mixture after reaction was successfully layered to two phases, with a transparent liquid containing no less than 96 wt% di- and tri-butyl glycerol ethers in the top phase as a product and a black mixture consisting of glycerol and SG in the bottom phase which can be used to start a new run with fresh isobutene addition. During six consecutive reaction–extraction cycles the catalyst maintained its robust performance.

 Please wait while we load your content...
Please wait while we load your content...