Room-temperature transfer hydrogenation and fast separation of unsaturated compounds over heterogeneous catalysts in an aqueous solution of formic acid†
Abstract
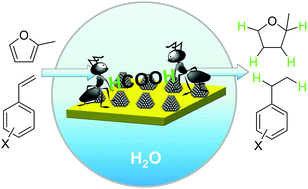
The facile conversion of olefins and unsaturated biomass to saturated compounds is achieved over heterogeneous catalysts composed of noble metal nanoparticles and carbon nitride. Reactions could proceed smoothly at room temperature in water using formic acid as the hydrogen source. The reusability of such a hybrid catalyst is high due to the strong Mott–Schottky effect between the metal nanoparticles and the carbon nitride support. The fast and automatic separation of the as-formed saturated hydrocarbons from water combined with the mild reaction conditions and the excellent reusability of catalysts make the catalytic process a highly “green” path for hydrogenation of unsaturated compounds and biofuel upgrading.

 Please wait while we load your content...
Please wait while we load your content...