Solvent-tuned hydrophobicity for faujasite-catalyzed cycloaddition of biomass-derived dimethylfuran for renewable p-xylene†
Abstract
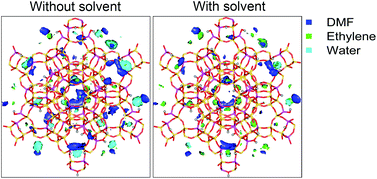
Phase equilibria of the high temperature/high pressure heterogeneous catalytic reaction of 2,5-dimethylfuran (DMF), a biomass-derived furan, with ethylene to produce p-xylene was studied as a function of DMF conversion in a constant-pressure reactor. Adsorption of reactants and products onto a zeolite catalyst was computed using a configurational-bias Grand Canonical Monte Carlo (CB-GCMC) simulation, and the vapor–liquid behavior was computed using ASPEN Plus with an appropriate equation of state. It was found that the amount of water adsorbed in H–Y (Si/Al = 2.6) increases significantly as DMF is consumed, but is small in the presence of an aliphatic solvent, here n-heptane, even at high DMF conversion. The presence of the solvent reduces side reactions with water, such as hydrolysis followed by oxyalkylation, and increases p-xylene selectivity, in agreement with experiment. The decrease in the amount of water adsorbed is due to the increased hydrophobic environment in the zeolite as a result of the addition of n-heptane. The increase of ethylene inside the cage by the addition of n-heptane results in a slight increase of p-xylene alkylation. This work presents the first use of molecular simulation to understand mechanistic effects of solvents on the catalytic production of p-xylene in an H–Y zeolite.


 Please wait while we load your content...
Please wait while we load your content...