Sulfur–silicon bond activation catalysed by Cl/Br ions: waste-free synthesis of unsymmetrical thioethers by replacing fluoride catalysis and fluorinated substrates in SNAr reactions†
Abstract
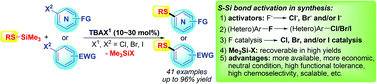
In contrast to conventional activation of Nu–SiR3 reagents by F ion attributed to the strong affinity of Si to F, S–Si activation can now be achieved using Cl/Br ions of TBAX as catalysts via formation of weaker X–Si bonds and Me3Si–X. This led to a waste-free synthesis of unsymmetrical thioethers via F-free SNAr reactions of activated (hetero)aryl halides and RS–SiMe3, with recovery of the useful Me3Si–X reagent in high yields.

 Please wait while we load your content...
Please wait while we load your content...