Synthesis of β-cyanopropan-1-one derivates by domino reaction†
Abstract
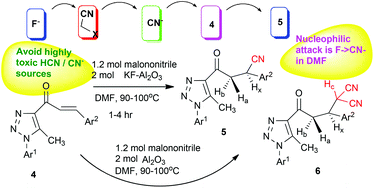
Domino nucleophilic addition was used for four-component Al2O3-catalyzed environmentally friendly synthesis of polysubstituted β-cyanopropan-1-one. Domino nucleophilic addition involves removal of the cyano group linked to active methylene by the action of KF, and direct addition to enones. The reaction's capability for nucleophilic attack is F− > CN− in DMF. The use of low-toxicity reagents hints that the reaction is more environmentally friendly than traditional approaches.

 Please wait while we load your content...
Please wait while we load your content...