Preparation of functional styrenes from biosourced carboxylic acids by copper catalyzed decarboxylation in PEG†
Abstract
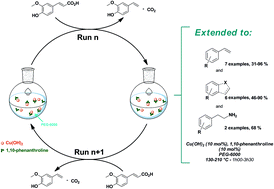
A general protocol for the copper-catalyzed decarboxylation of α,β-unsaturated carboxylic acids in PEG, particularly of biosourced cinnamic acid analogues, is reported. Moderate to high isolated yields (31–96%) towards the styrene derivatives were obtained. For the first time, decarboxylation of α-amino acids to the corresponding amines was successfully performed with good to high yields and extended to the decarboxylation of a few condensed heterocyclic compounds. Both the use of PEG as a green solvent and direct separation of the pure product of the reaction by distillation permitted the reuse of the solvent and the Cu-based catalytic system over several cycles without deactivation. This was extended to the synthesis of 4-vinylguaiacol on the laboratory scale in an average 92% yield.

 Please wait while we load your content...
Please wait while we load your content...