Enhanced laccase stability through mediator partitioning into hydrophobic ionic liquids
Abstract
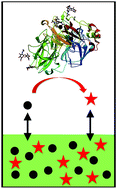
Laccase-mediator systems have numerous potential uses for green oxidations, but their practical use may be limited because the reactive, oxidised mediators deactivate the enzyme. TEMPO, 4-hydroxybenzyl alcohol, phenothiazine and 2-hydroxybiphenyl caused almost complete deactivation of laccase from Trametes versicolor within 24–140 h. By contrast, 18% activity was retained after 188 h in controls without mediator, and 15% in the presence of ABTS. A biphasic reaction system was developed to protect the laccase, by partitioning the mediator into water-immiscible ionic liquids. In the presence of [C6mim][AOT], laccase retained 54, 35, 35 and 41% activity after 188 h in the presence of 4-hydroxybenzyl alcohol, phenothiazine and 2-hydroxybiphenyl and ABTS, respectively, whilst 30% activity was retained in the presence of [N1 8 8 8][Sac] and TEMPO. The protection against deactivation by the mediators correlated strongly with the distribution coefficients of the mediators between ionic liquids and water.

 Please wait while we load your content...
Please wait while we load your content...