Sustainable and selective synthesis of 3,4-dihydroquinolizin-2-one and quinolizin-2-one derivatives via the reactions of penta-3,4-dien-2-ones†
Abstract
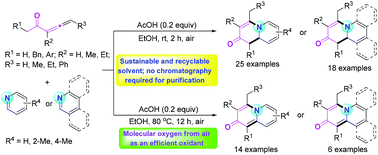
In this paper, a novel and green methodology for the synthesis of 3,4-dihydroquinolizin-2-one derivatives via the tandem reactions of penta-3,4-dien-2-ones with pyridine, quinoline, or isoquinoline at rt in aqueous ethanol was developed. Notably, the reactions involved a convenient work-up of simple precipitation and filtration, and the solvent could be easily recycled. In addition, when the reactions were carried out at higher temperature over a prolonged reaction period, the in situ formed 3,4-dihydroquinolizin-2-ones could be dehydrogenated by molecular oxygen from air to afford quinolizin-2-ones with good efficiency.

 Please wait while we load your content...
Please wait while we load your content...