Comparison of the influence of a Lewis acid AlCl3 and a Brønsted acid HCl on the organosolv pulping of beech wood†
Abstract
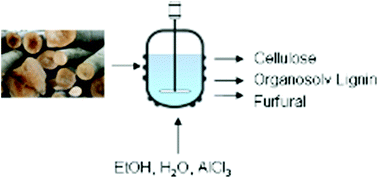
In this work the influence of a Lewis acid AlCl3 and a Brønsted acid HCl on the organosolv process is investigated. Beech wood is pulped with an aqueous ethanol solution (50 vol%) for 1 h in the temperature range from 150 °C to 190 °C. Also the organosolv lignins are investigated regarding their molar mass distribution and OH-groups occurrence. The xylan degradation kinetics of the AlCl3 catalyzed pulping is investigated at 170 °C and compared with literature data. For this purpose kinetic models from the literature are used. The activation energy of xylan hydrolysis was determined as 61 kJ mol−1. This value is much lower than other reported activation energies using Brønsted acids as catalysts.

 Please wait while we load your content...
Please wait while we load your content...