Catalyst-free chemo-/regio-/stereo-selective amination of alk-3-ynones. Synthesis of 1,5-benzodiazepines and 3-amino-2-alkenones†
Abstract
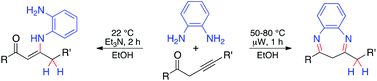
Reaction of alk-3-yn-1-ones with o-phenylenediamines provides an effective method with high atom economy for the synthesis of diversely substituted benzodiazepines and conjugated enaminones. This microwave-accelerated reaction proceeds in ethanol in the absence of a catalyst and leads to benzyl-substituted 1,5-benzodiazepines with good yields (70–92%). A room temperature protocol with the same set of reagents (stabilized with triethylamine) leads to enaminones (3-amino-2-alkenones, 70–99%). The tautomer formed and the regio- and stereochemistry of the process are confirmed by the X-ray crystallographic structure determination of 2-(4-methylbenzyl)-4-phenyl-3H-benzo[b][1,5]diazepine and (Z)-3-[(2-amino-4,5-dimethylphenyl)amino]-4-(4-tert-butylphenyl)-1-(4-chlorophenyl)but-2-en-1-one.

 Please wait while we load your content...
Please wait while we load your content...