Stereodivergent Michael addition of diphenylphosphite to α-nitroalkenes in the presence of squaramide-derived tertiary amines: an enantioselective organocatalytic reaction in supercritical carbon dioxide†
Abstract
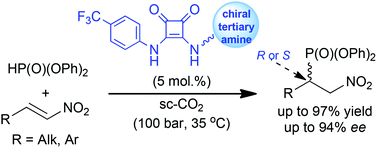
The first “green” asymmetric organocatalytic reaction in a supercritical carbon dioxide medium was elaborated. Under the proposed conditions (100 bar, 35 °C), α-nitroalkenes enantioselectively accept diphenylphosphite in the presence of bifunctional organocatalysts bearing the tertiary amino group and the squaramide fragment to afford corresponding β-nitrophosphonates in high yields and with enantioselectivities of up to 94% ee. By varying the catalyst structure it is possible to synthesize both β-nitrophosphonate enantiomers under these conditions. A significant potential of the supercritical extraction for product isolation and catalyst recovery was demonstrated.

 Please wait while we load your content...
Please wait while we load your content...