Coordination effect-regulated CO2 capture with an alkali metal onium salts/crown ether system†
Abstract
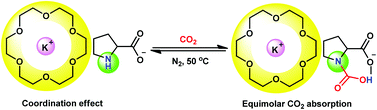
A coordination effect was employed to realize equimolar CO2 absorption, adopting easily synthesized amino group containing absorbents (alkali metal onium salts). The essence of our strategy was to increase the steric hindrance of cations so as to enhance a carbamic acid pathway for CO2 capture. Our easily synthesized alkali metal amino acid salts or phenolates were coordinated with crown ethers, in which highly sterically hindered cations were obtained through a strong coordination effect of crown ethers with alkali metal cations. For example, a CO2 capacity of 0.99 was attained by potassium prolinate/18-crown-6, being characterized by NMR, FT-IR, and quantum chemistry calculations to go through a carbamic acid formation pathway. The captured CO2 can be stripped under very mild conditions (50 °C, N2). Thus, this protocol offers an alternative for the development of technological innovation towards efficient and low energy processes for carbon capture and sequestration.

 Please wait while we load your content...
Please wait while we load your content...