Decarbonylation of heptanoic acid over carbon-supported platinum nanoparticles
Abstract
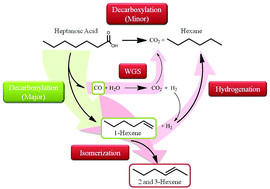
The decarbonylation and decarboxylation of heptanoic acid over carbon-supported Pt nanoparticles were studied in a continuous flow fixed bed reactor at 573 K and 37 bar for liquid-phase operation and 1 bar for gas-phase operation. Under liquid-phase conditions, the TOF over Pt supported on Norit carbon was 0.0052 s−1 and independent of Pt loading. At very low conversions, approaching zero, the product selectivity was consistent with decarbonylation as the primary reaction, producing mostly hexenes and CO. As conversion increased from 1% to 5% at 37 bar, substantial amounts of hexane and CO2 were observed, presumably from secondary side reactions such as water-gas shift (WGS) and hydrogenation instead of direct decarboxylation. The terminal olefin was observed with high selectivity (57%) only during gas-phase operation (1 bar) which facilitated transport of the olefin away from the Pt that also catalyzed double bond isomerization. Some sintering of the Pt metal particles during reaction of heptanoic acid was observed by X-ray diffraction analysis of the spent catalyst. Catalyst regeneration studies were performed over spent catalyst but they failed to restore any catalytic activity.
- This article is part of the themed collection: Conversion of biomass with heterogeneous catalysts

 Please wait while we load your content...
Please wait while we load your content...