An efficient palladium catalyst on bentonite for Suzuki–Miyaura reaction at room temperature†
Abstract
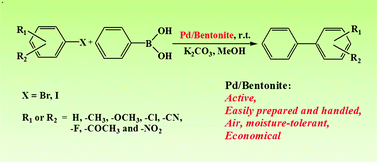
Clays, which are nontoxic, abundant, and cheap, are very promising supports for the design and preparation of green catalysts. In this work, the Pd/bentonite catalyst was fabricated by a simple impregnation method using water as the medium. The catalyst was characterized by powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), transmission electron spectroscopy (TEM), X-ray photoelectron (XPS) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) techniques. The performance of Pd/bentonite in the Suzuki–Miyaura reaction was studied. It was found that for aryl bromides and iodides with various electron-donating and electron-withdrawing groups such as –CH3, –OCH3, –Cl, –CN, –F, –COCH3 and –NO2, the coupling reaction of substrates with arylboronic acid proceeded smoothly at low catalyst loading (Pd 0.06 mol%) under ambient temperature. The catalyst could be reused at least 7 times without any decrease in activity.

 Please wait while we load your content...
Please wait while we load your content...