Direct oxidation of secondary alcohol to ester by performic acid†
Abstract
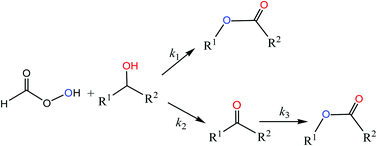
The reaction pathways and kinetics of the oxidation reactions of 1-phenylethyl alcohol (PEA), 1-(3,4-dimethoxyphenyl)ethanol (MVA), 1-(4-hydroxy-3-methoxyphenyl)ethanol (HMOPE), 1-(3-aminophenyl)ethanol (APE), 1-(4-methylphenyl)ethanol (MPE) and cyclohexanol with performic acid (PFA) were investigated in formic acid solvent. An unexpected new reaction pathway, from which the secondary alcohols can be directly oxidized to corresponding esters, was found. The reaction products (esters and ketones) of oxidation of PEA, MVA, HMOPE, APE, MPE and cyclohexanol were detected at different reaction times. The reaction rate constants, k1, k2, k3 and power orders α, β, γ of PFA concentration for three oxidation reactions pathways: alcohol to ester, alcohol to ketone and ketone to ester were obtained, respectively. These findings might provide a new insight into the technology of lignin degradation.

 Please wait while we load your content...
Please wait while we load your content...