Metal-free arylations via photochemical activation of the Ar–OSO2R bond in aryl nonaflates†
Abstract
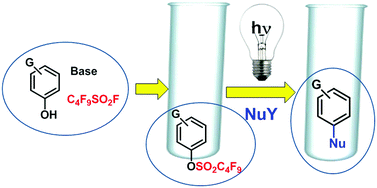
The photolysis of electron-rich aryl nonaflates (ArONfs) in protic media was investigated and heterolysis of the Ar–OS bond (from 3ArONf) took place. The reaction generated a triplet phenyl

 Please wait while we load your content...
Please wait while we load your content...