Base initiated depolymerization of polycarbonates to epoxide and carbon dioxide co-monomers: a computational study†
Abstract
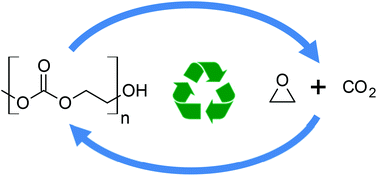
High-accuracy CBS-QB3(+) calculations were used to obtain the free energy barriers for several polycarbonates of interest to undergo alkoxide back-biting to give the corresponding

 Please wait while we load your content...
Please wait while we load your content...