Mesoporous poly-melamine-formaldehyde (mPMF) – a highly efficient catalyst for chemoselective acetalization of aldehydes†
Abstract
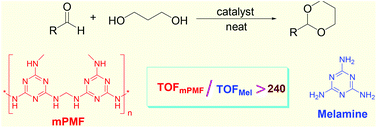
A mesoporous poly-melamine-formaldehyde polymer with a high surface area, good porosity and a high density of amine and triazine functional groups was investigated as a highly efficient hydrogen-bonding catalyst. This porous organic polymer was found to be highly effective in catalyzing chemoselective acetalization of aldehydes, without the consumption of any dehydrating agents. The turnover frequency of mesoporous poly-melamine-formaldehyde is hundreds of times higher than melamine monomer, and this high efficiency is due to the high density of aminal (–NH–CH2–NH–) groups and triazine rings in the polymer network, which provides an inherently powerful system with multiple hydrogen bonds. This unique characteristic imparts mesoporous poly-melamine-formaldehyde polymer with a very high activity as a heterogeneous organocatalyst. The polymer is also low cost, and easy to be synthesized and recycled.

 Please wait while we load your content...
Please wait while we load your content...