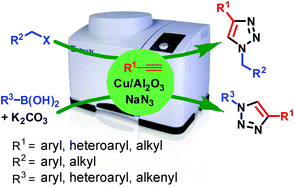
A one-pot procedure for the synthesis of 1,2,3-triazole derivatives by a three-component coupling of alkyl (benzyl) halides or aryl boronic acids, sodium azide and terminal alkynes over copper(II) sulfate supported on alumina (Cu/Al2O3) under ball-milling in the absence of any solvent and additive has been developed. The product was isolated by simple washing of the crude reaction residue with ethanol followed by evaporation of the solvent. No chromatographic purification is required. The catalyst is recycled for subsequent reactions. The azides are produced in situ and thus this procedure avoids the handling of hazardous azides. This protocol offers broad scope for access to a variety of diversely substituted 1,2,3-triazoles. The use of no hazardous organic solvent, the use of ball-milling, and cost efficiency, recyclability of the catalyst up to eight runs without appreciable loss of activity and high yields of products make this procedure greener.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...