A cobalt Schiff base with ionic substituents on the ligand as an efficient catalyst for the oxidation of 4-methyl guaiacol to vanillin†
Abstract
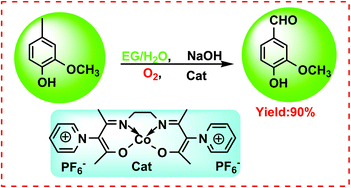
A cobalt Schiff base catalyst with ionic substituents on the ligand, N,N′-ethylenebis(acetylacetoniminato)-cobalt(II) hexafluorophosphoric pyridinium (Co-[Salen-Py][PF6]2), was synthesized. It displayed an excellent catalytic performance for the oxidation of 4-methyl guaiacol to vanillin (conversion = 100%, selectivity = 90%). Tentative reaction mechanism research indicated that the electron-withdrawing pyridinium substituent on the ligand of Co(acacen) is responsible for the high selectivity of vanillin. Meanwhile, utilizing ethylene glycol and water as solvent, vanillin can be isolated by simple crystallization in the form of a sodium salt, and the mother liquid of the crystallization, with a large amount of NaOH (the mole ratio of NaOH/4-methyl guaiacol = 2.38/1), can be successfully recycled at least three times, thereby decreasing the mole ratio of base/substrate from 3.3 : 1 to 1.05 : 1 when the mother liquid of crystallization was recycled. This strategy provides a potentially greener alternative for the synthesis of vanillin in industry.

 Please wait while we load your content...
Please wait while we load your content...