Catalytic procedures for multicomponent synthesis of imidazoles: selectivity control during the competitive formation of tri- and tetrasubstituted imidazoles
Abstract
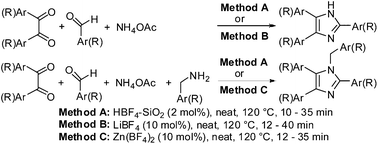
The catalytic potential of different fluoroboric acid-derived catalyst systems viz. aq HBF4, solid supported HBF4, metal tetrafluoroborates (inorganic salts), solid supported metal tetrafluoroborates, and tetrafluoroborate based ionic liquids (organic salts) were investigated for the three component reaction (3-MCR) of 1,2-diketone, aldehyde, and ammonium salts to form 2,4,5-trisubstituted imidazoles and the four component reaction (4-MCR) involving 1,2-diketone, aldehyde, amine and ammonium acetate to form 1,2,4,5-tetrasubstituted imidazoles. The HBF4–SiO2 was found to be the stand out catalyst for both the 3-MCR and 4-MCR processes. The next most effective catalysts are LiBF4 and Zn(BF4)2 to form 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles via the 3-MCR and 4-MCR, respectively. This is the first report on the unaddressed issue of competitive formation of 2,4,5-trisubstituted imidazole during the 4-MCR involving 1,2-diketone, aldehyde, amine and ammonium acetate and highlights the influence of the catalyst systems in controlling the selective formation of tetra substituted imidazole. The metal salt of weak protic acids drive selectivity towards tetra substituted imidazole in the order tetrafluoroborates > perchlorates > triflates. The catalytic potency of tetrafluoroborates was in the order Zn(BF4)2 > Co(BF4)2 > AgBF4 ≈ Fe(BF4)2 > NaBF4 ≈ LiBF4 ≈ Cu(BF4)2. The developed protocols worked well for different diketones, various aryl, heteroaryl, and alkyl aldehydes and in the case of the preparation of 1,2,4,5-tetrasubstituted imidazoles different amines can be used. The effectiveness of different ammonium salts as nitrogen source has been investigated and ammonium acetate is proved to be the best. The HBF4–SiO2 is recyclable for five consecutive uses without significant loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...