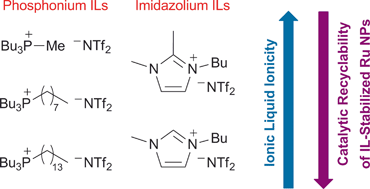
Ruthenium nanoparticles (Ru NPs) were synthesized from the reduction of Ru(2-methylallyl)2(cod) under an atmosphere of H2(g) in a series of phosphonium and imidazolium ionic liquids (ILs): [P4,4,4,1]NTf2, [P4,4,4,8]NTf2, [P4,4,4,14]X (for −X = −NTf2, −OTf, −PF6), [BMI]NTf2 and [BDMI]NTf2. The Ru NPs embedded in each of these ILs were used as biphasic hydrogenation catalysts for the reduction of cyclohexene. The properties of the NPs, in terms of NP stability and catalytic activity, were dependent on the nature of the IL and these differences were rationalized through a Walden plot analysis of the IL ionicity. This analysis supports the idea that the formation of ion associations, in terms of ion-pairs or supramolecular aggregates, plays a key role in IL stabilization of metal NPs. A correlation between this stabilization mechanism and the NP stability and catalytic activity is hypothesized.

 Please wait while we load your content...
Please wait while we load your content...