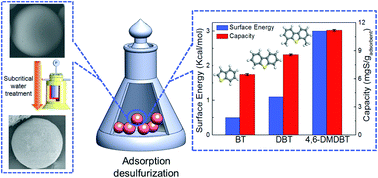
This paper explores porous glass beads as a pollutant-free-prepared, low-cost, and recyclable adsorbent for desulfurization. Porous glass beads with a specific surface area, pore volume, and mean pore diameter of 162.6 m2 g−1, 0.26 cm3 g−1, and 6.34 nm, respectively, were obtained by subcritical water treatment. The effects of the surface chemistry of the adsorbent and the structure of the organosulfur molecules on adsorption capacity were studied; the software, Materials Studio, was used to calculate the interactions between the surface of the glass beads and the sulfur-containing compounds. Compared with these porous glass beads, two other kinds of porous glass beads modified by hexamethyldisilazane and hydrochloric acid, respectively, showed much lower adsorptive capacity for dibenzothiophene, indicating the metal ions contained in the glass played an important role during the adsorption; the capacities of the porous glass beads for benzothiophene, dibenzothiophene, and 4,6-dimethyldibenzothiophene were 6.47 ± 0.09, 8.58 ± 0.09, and 11.20 ± 0.08 mg(S) gadsorbent−1, respectively, corresponding to the simulation results. The adsorptive capacity for dibenzothiophene decreased with the increase of temperature but increased with the increase of initial concentration, the highest capacity of 10.29 ± 0.11 mg(S) gadsorbent−1 was obtained at 303 K with the initial concentration of 565 ppmw(S). Spent adsorbent can be regenerated by heating at high temperature, and the adsorptive capacity only decreased about 3.38% after five cycles. Experimental results and the computer simulations indicate that polar interactions between the surface and sulfur-containing compounds dominated the adsorption.

 Please wait while we load your content...
Please wait while we load your content...