Domino reactions in water: diastereoselective synthesis of densely functionalized indolyldihydrofuran derivatives†
Abstract
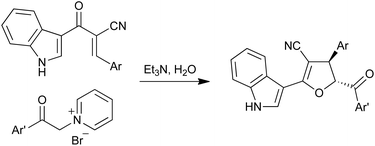
A library of trans-5-aroyl-2-(indol-3-yl)-4-aryl-4,5-dihydrofuran-3-carbonitriles was diastereoselectively synthesized in excellent yields from the reaction of 2-(3-indolylcarbonyl)-3-aryl-2-propenenitriles with (2-aryl-2-oxoethyl)pyridinium bromides in the presence of

 Please wait while we load your content...
Please wait while we load your content...