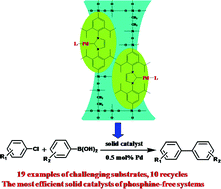
An IPr-functionalized (IPr = N,N′-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, a N-heterocyclic carbene ligand) mesoporous ethane-silica was synthesized through a co-condensation of IPr-bridged triethoxysilane and bis(triethoxysilyl)ethane. This functionalized mesoporous ethane-silica was characterized with N2 sorption, XRD, FT-IR, solid NMR and XPS. Owing to high surface area, large pore volume and organically functionalized surface, such a material can be used as a scaffold to design surface NHC-Pd [NHC = N-heterocyclic carbene] complexes. By variation of Pd precursors and/or ancillary ligands, a library of NHC-Pd complexes on the solid surface were tentatively created. It was found that the Pd precursor and ancillary ligands had significant impacts on the activities of the resultant heterogeneous catalysts in the Suzuki–Miyaura coupling of aryl chlorides. Surface NHC-Pd complexes with acetylacetonate (acac) and 3-chloropyridine as ancillary ligands are the most active. A wide range of aryl chlorides even with deactivated and sterically hindered groups were successfully coupled over this solid catalyst under the mild conditions at a relatively low loading of Pd (80 °C, 0.5 mol% Pd). This solid catalyst could be reused 10 times without a significant decrease in activity. This study not only provides the most efficient phosphine-free solid catalyst for the Suzuki–Miyaura couplings of less reactive substrates, but also demonstrates a noteworthy example that IPr-functionalized ethane-silica can be used as a versatile scaffold to create various active surface NHC-metal catalysts.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...