Acid promoted CIDT for the deracemization of dihydrocinnamic aldehydes with Betti's base†
Abstract
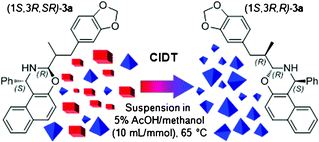
Racemic α-epimerizable and unfunctionalized aldehydes have been converted into enantiomerically enriched mixtures through a sequence of (i) a conversion into the diastereoisomeric 3-substituted 1-phenyl-2,3-dihydro-1H-naphtho[1,2-e][1,3]oxazines by reaction with the (R)- or

 Please wait while we load your content...
Please wait while we load your content...