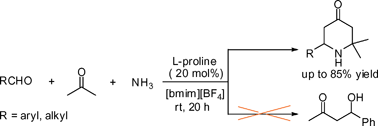
Highly efficient chemoselective construction of 2,2-dimethyl-6-substituted 4-piperidones via multi-component tandem Mannich reaction in ionic liquids†
Abstract
The room temperature ionic liquid [bmim][PF6] has been demonstrated to be an efficient and recyclable medium for highly chemoselective synthesis of

 Please wait while we load your content...
Please wait while we load your content...