To contribute to a deeper insight into the hazard potential of ionic liquids to humans and the environment, an acetylcholinesterase (AchE) inhibition screening assay was used to identify toxicophore substructures and interaction potentials mediating enzyme inhibition.
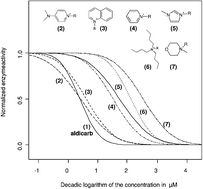
The positively charged nitrogen atom, a widely delocalised aromatic system, and the lipophilicity of the side chains connected to the cationic head groups can be identified as the key structural elements in binding to the enzymes active site. With respect to this, the dimethylaminopyridinium, the quinolinium and the pyridinium head groups exhibit a very strong inhibitory potential to the enzyme with IC50 values around 10 µM. In contrast, the polar and non-aromatic morpholinium head group is found to be only weakly inhibiting to the enzyme activity, with IC50 values > 500 µM.
The introduction of polar hydroxy, ether or nitrile functions into the alkyl side chain is shown to be a potent structural alteration to shift the corresponding ionic liquids to a lower inhibitory potential. Supporting this fact, for a series of imidazolium cations, a QSAR correlation was set up by the linear regression of the log IC50versus the logarithm of the HPLC-derived lipophilicity parameter k0.
Additionally, a broad set of anion species (inorganic, organic and complex borate anions), commonly used as ionic liquid counterions, was tested and the vast majority exhibited no effect on AchE. Only the fluoride and fluoride containing anion species which readily undergo hydrolytic cleavage can be identified to act as AchE inhibitors.

 Please wait while we load your content...
Please wait while we load your content...