Electrocatalytic conversion of CO2 to long carbon-chain hydrocarbons†
Abstract
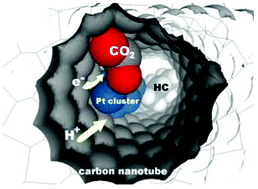
It is shown for the first time that using Pt nanoparticles on carbon-based electrodes it is possible to convert CO2 to long carbon-chain hydrocarbons (>C5) at room temperature and atmospheric pressure in a continuous flow cell having the working electrode directly in contact with the CO2 in the gas phase. The performances and product distribution depend on the nature of the electrocatalyst and the reaction conditions. It is also shown that product distribution is different from that expected from Anderson–Schultz–Flory distribution for Fischer–Tropsch synthesis. This step integrates in a photoelectrochemical device to ultimately use solar energy and water to convert back CO2 to fuels. The possibility of the use of this device for Mars missions is also mentioned.
- This article is part of the themed collection: Green chemistry for fuel synthesis and processing

 Please wait while we load your content...
Please wait while we load your content...