An effective synthesis of bromoesters from aromatic aldehydes using tribromide ionic liquid based on l-prolinol as reagent and reaction medium under mild conditions†
Abstract
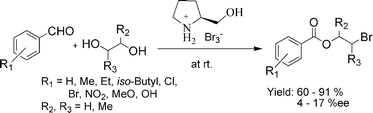
Two kinds of ionic liquids have been directly synthesized from L-prolinol by a simple and convenient method in excellent yields. The application of the ionic liquids as reagents and solvents for the chemoselective, regioselective, and stereoselective synthesis of 1,2- or 1,3-bromoesters from aromatic aldehydes and 1,2- or 1,3-diols at room temperature has been studied. Good to excellent yields and moderate enantiomeric excesses were obtained under these reaction conditions

 Please wait while we load your content...
Please wait while we load your content...