Clean Beckmann rearrangement of cyclohexanone oxime in caprolactam-based Brønsted acidic ionic liquids†‡
Abstract
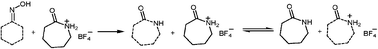
The Beckmann rearrangement of cyclohexanone oxime to afford caprolactam in a novel caprolactam-based Brønsted acidic ionic liquid as catalyst and reaction medium proceeded with high conversion and selectivity at 100 °C. The occurrence of the Beckmann rearrangement of cyclohexanone oxime in such a Brønsted acidic IL was also confirmed with in situ FT-Raman observation. The key point is that the caprolactam product was one component of the ionic liquid, and a dynamic exchange between the resulting caprolactam product and the caprolactam from the ionic liquid is expected. Therefore, the strong chemical combination between the caprolactam product and the acidic ionic liquid was greatly decreased and the desired product in the solid was recovered through extraction with organic solvent after the reaction.

 Please wait while we load your content...
Please wait while we load your content...