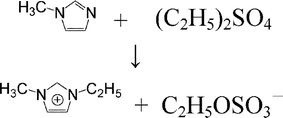
This paper reports measurements of the surface tension of ionic liquid EMISE (1-ethyl-3-methylimidazolium ethyl sulfate) using the forced bubble method at 278.15 to 323.15 K and densities of EMISE using a Westphal balance at 278.15 to 338.15 K. At the same time, a new theoretical model, the interstice model, is put forward. Applying the model, an expression of the average volume of the interstices, v, was obtained. The calculated volume fraction of the total interstices is 0.12 for ionic liquid EMISE and is in good agreement with that of the majority of materials which exhibit a 10∼15% volume expansion in transition from the solid to liquid state. The value of the thermal expansion coefficient calculated from the model is 5.24 × 10−4 K−1 and is in good agreement with the experimental value 5.37 × 10−4 K−1 at 298.15 K.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...