Reforming of methane by CO2 in presence of cobalt- based catalysts†
Abstract
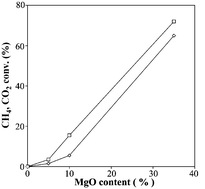
Reforming of methane by CO2 to syngas has been studied on cobalt-based catalysts. The catalysts have been characterised by BET, CO2 adsorption and TG-DSC techniques. The catalytic activity and stability of catalysts are closely related to the basic character of the magnesium oxide additive. It has been observed that the catalytic activity increases with the MgO loading. The order of activity is the following: Co/SiO2 < Co/5 wt% MgO–SiO2 < Co/10 wt% MgO–SiO2 < Co/35 wt% MgO–SiO2. Moreover, the positive effect of MgO on the resistance to carbon deposition by adjustment of the acid–base function of the support has been evidenced. The basic function of the metal oxide promoter also seemed to increase H-abstraction of methane and CO2 adsorption.

 Please wait while we load your content...
Please wait while we load your content...