EPZ-10 catalyzed regioselective transformation of alkenes into β-iodo ethers, iodohydrins and 2-iodomethyl-2,3-dihydrobenzofurans
Abstract
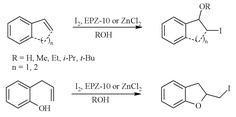
An efficient method for the regioselective synthesis of β-iodo ethers and iodohydrines from styrene, indene and dihydronaphthalene in presence of EPZ-10R has been presented wherein only 0.5 equivalent iodine (I2) has been used. Similarly, o-allylphenols are converted to 2-iodomethyl-2,3-dihydrobenzofurans in high yields. This demonstrates a clean green chemical transformation with significant atom economy.

 Please wait while we load your content...
Please wait while we load your content...