Indium and tin-mediated allylation in ionic liquids
Abstract
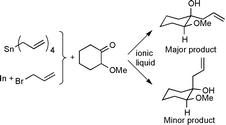
The first report of the use of indium metal and allyl bromide for the allylation of aldehydes and ketones in ionic liquids is given in this paper. The homoallylic alcohol products are obtained in high yields on reaction at room temperature using stoichiometric quantities of indium. Initial results gained using a catalytic system of indium, manganese and TMSCl are also reported. The effect of ionic liquid solvents on the stereochemical selectivity of allylation of 2-methoxycyclohexanone and benzoin methyl ether has been investigated, showing a higher selectivity towards the chelation-controlled mechanism in ionic liquids than in conventional solvents such as water and THF. Using tetraallyltin as the allylating agent, the same reaction occurs with modest improvements in stereoselectivity compared with conventional solvents. The addition of 5 mol% Sc(OTf)3, however, results in a large increase in both reaction rate and selectivity, and permits the isolation of high yields of product via a simple workup procedure. The ionic liquid/Sc(OTf)3 mixture may also easily be recycled with good maintenance of yield and stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...