On the solubilization of water with ethanol in hydrophobic hexafluorophosphate ionic liquids
Abstract
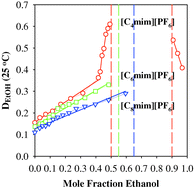
The solubility of water in the hydrophobic 1-alkyl-3-methylimidazolium hexafluorophosphate (alkyl = butyl, hexyl, and octyl) ionic liquids, can be significantly increased in the presence of ethanol as a co-solute. 1-Hexyl-3-methylimidazolium hexafluorophosphate and 1-octyl-3-methylimidazolium hexafluorophosphate are completely miscible with ethanol, and immiscible with water, whereas 1-butyl-3-methylimidazolium hexafluorophosphate is totally miscible with aqueous ethanol only between 0.5–0.9 mole fraction ethanol at 25 °C. At higher and lower mole fraction of ethanol, the aqueous and IL components are only partially miscible and a biphasic system is obtained upon mixing equal volumes of the IL and aqueous ethanol. The observation of a large range of total miscibility between water and the IL in the three-component system has important implications for purifications and separations from IL.

 Please wait while we load your content...
Please wait while we load your content...