Inhibitory mechanism of epicatechin gallate on tyrosinase: inhibitory interaction, conformational change and computational simulation
Abstract
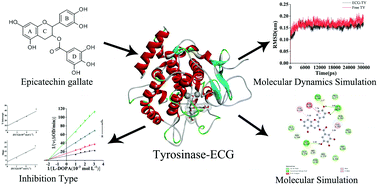
The inhibition mechanism of epicatechin gallate (ECG) on tyrosinase was investigated by multispectroscopic techniques combined with molecular docking and molecular dynamics simulation. The results demonstrated that ECG suppressed the activity of tyrosinase in a reversible mixed-inhibition with a half inhibitory concentration (IC50) of (1.13 ± 0.82) × 10−5 mol L−1. Binding of ECG to tyrosinase led to the formation of a complex with the binding constant (Ksv) of 4.03 × 104 L mol−1 at 298 K which was stabilized by hydrophobic forces. The complex formation induced the intrinsic fluorescence quenching and secondary structure change of tyrosinase. Molecular docking results showed that hydrophobic and hydrogen bonding forces played a dominant role in the binding of ECG to tyrosinase, affecting the binding affinity of L-dopa to tyrosinase, leading to a decrease in tyrosinase activity. Molecular dynamics analysis indicated that ECG led to the stretching of the basic framework structure of tyrosinase and slightly influenced the microenvironment of amino acid residues. The research might provide new perspectives on the inhibition mechanism of epicatechin gallate on tyrosinase and a theoretical basis for the prevention and treatment of pigmented skin diseases and anti-browning of catechin as a food supplement.


 Please wait while we load your content...
Please wait while we load your content...