Exploring the structure–activity relationship and interaction mechanism of flavonoids and α-glucosidase based on experimental analysis and molecular docking studies†
Abstract
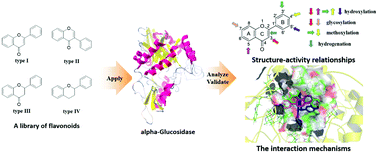
α-Glucosidase (AG) has always been an indispensable drug target for the treatment of type 2 diabetes. Herein, an integrated method consisting of enzyme kinetics, multi-spectroscopy assay and molecular simulations was used to investigate the structure–activity relationship and interaction mechanism of flavonoids and AG. As a result, a small amount of flavonoids was found to present excellent inhibitory activity on AG, such as 3, 5, 6, 8, 10, 17, 19, 21, 22 and 34. Further analysis of the structure–activity relationship illustrated that hydroxylation at the positions C3, C6, C3′ and C4′ of flavonoids caused an increase in the inhibitory activity of AG, whereas the methoxylation at the corresponding positions would decrease the activity. Also, it was found that the glycosylation and hydrogenation of the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 double bond would distinctly reduce the inhibition potency. Therefore, various groups at different positions of flavonoids exhibited an upward and downward tendency in the activity. According to the fluorescence quenching assay, all of the test flavonoids could effectively quench the intrinsic fluorescence of AG based on either the static or mixed static–dynamic mechanism. Besides, the thermodynamic parameters of the representative flavonoid 19 revealed the spontaneous characteristic of the binding process with AG, and highlighted the critical role of the hydrophobic interaction and hydrogen bonds. Moreover, the results obtained from the synchronous fluorescence, ANS-binding fluorescence, Fourier transform infrared and circular dichroism spectra illustrated that these active flavonoids could bind to the active site of AG and induce the rearrangement and conformation change of its secondary structures, which resulted in a significant inhibitory activity. Additionally, molecular modelling visualized the preferred binding conformation of flavonoids on AG, and further confirmed the great importance of the hydrophobic interaction and hydrogen bonds in the interaction. Such findings provided new insights for understanding the proposed interaction behavior between flavonoids and AG, and were helpful to develop novel AG inhibitors relying on the flavonoid scaffold for the treatment of type 2 diabetes.
C3 double bond would distinctly reduce the inhibition potency. Therefore, various groups at different positions of flavonoids exhibited an upward and downward tendency in the activity. According to the fluorescence quenching assay, all of the test flavonoids could effectively quench the intrinsic fluorescence of AG based on either the static or mixed static–dynamic mechanism. Besides, the thermodynamic parameters of the representative flavonoid 19 revealed the spontaneous characteristic of the binding process with AG, and highlighted the critical role of the hydrophobic interaction and hydrogen bonds. Moreover, the results obtained from the synchronous fluorescence, ANS-binding fluorescence, Fourier transform infrared and circular dichroism spectra illustrated that these active flavonoids could bind to the active site of AG and induce the rearrangement and conformation change of its secondary structures, which resulted in a significant inhibitory activity. Additionally, molecular modelling visualized the preferred binding conformation of flavonoids on AG, and further confirmed the great importance of the hydrophobic interaction and hydrogen bonds in the interaction. Such findings provided new insights for understanding the proposed interaction behavior between flavonoids and AG, and were helpful to develop novel AG inhibitors relying on the flavonoid scaffold for the treatment of type 2 diabetes.


 Please wait while we load your content...
Please wait while we load your content...