Supply of methionine and arginine alters phosphorylation of mechanistic target of rapamycin (mTOR), circadian clock proteins, and α-s1-casein abundance in bovine mammary epithelial cells†
Abstract
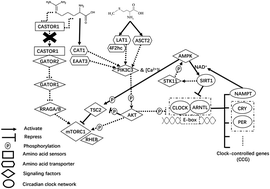
Methionine (Met) and arginine (Arg) regulate casein protein abundance through alterations in activity of the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway. A potential role for the circadian clock network on the regulation of protein synthesis, partly via activity of mTORC1, has been highlighted in non-ruminants. The main objective of the study was to determine in ruminant mammary cells alterations in mRNA, protein abundance and phosphorylation status of mTORC1-related upstream targets, circadian clock proteins, and protein kinase AMP-activated catalytic subunit alpha (AMPK) in relation to α-s1-casein protein (CSN1S1) abundance in response to greater supply of Met and Arg alone or in combination. Primary bovine mammary epithelial cells (BMEC) were incubated for 12 h in a 2 × 2 arrangement of treatments with control media (ideal profile of amino acids, IPAA), or media supplemented with increased Met (incMet), Arg (incArg), or both (incMet + incArg). Data were analyzed testing the main effects of Met and Arg and their interaction. Among 7 amino acid (AA) transporters known to be mTORC1 targets, increasing supply of Arg downregulated SLC1A5, SLC3A2, SLC7A1, and SLC7A5, while increasing supply of Met upregulated SLC7A1. mRNA abundance of the cytosolic Arg sensor (CASTOR1) was lower when supply of Arg and Met alone increased. p-TSC2 (TSC complex subunit 2) was greater when the Arg supply was increased, while the phosphoralation ratio of p-AKT (AKT serine/threonine kinase 1):total (t) AKT and p-AMPK:tAMPK were lower. In spite of this, the ratio of p-mTOR:tmTOR nearly doubled with incArg but such response did not prevent a decrease in CSN1S1 abundance. The abundance of period circadian regulator 1 (PER1) protein nearly doubled with all treatments, but only incMet + incArg led to greater clock circadian regulator (CLOCK) protein abundance. Overall, data suggest that a greater supply of Met and Arg could influence CSN1S1 synthesis of BMEC through changes in the mTORC1, circadian clock, and AMPK pathways. Identifying mechanistic relationships between intracellular energy, total AA supply, and these pathways in the context of milk protein synthesis in ruminants merits further research.
- This article is part of the themed collection: Food & Function Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...