A study towards drug discovery for the management of type 2 diabetes mellitus through inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by chalcone derivatives
Abstract
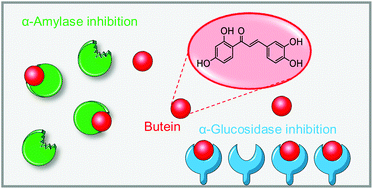
The inhibition of carbohydrate-hydrolyzing enzymes, α-amylase and α-glucosidase, is one of the major therapeutic strategies for the treatment of type 2 diabetes mellitus. Chalcones have been recognized for their multiple biological activities, including antidiabetic properties, through unclear mechanisms. In the present work, a panel of chalcones bearing hydroxy, methoxy, methyl, nitro, chloro, fluoro and bromo substituents were evaluated against α-amylase and α-glucosidase activities, most of them for the first time. The results showed that the substitution patterns and the type of substituents of chalcones influence their inhibitory activity. The presence of hydroxy groups at C-2′- and C-4′ of the A ring and at C-3 and C-4 of the B ring favors the intended effect. Chalcones holding nitro groups and chloro substituents, together with a hydroxy group in the chalcone scaffold, showed strong inhibition of the α-glucosidase activity. The present study provides related scaffolds that may serve as the basis for the design and synthesis of new structures in order to obtain the ideal antidiabetic chalcone.

 Please wait while we load your content...
Please wait while we load your content...