Protein–polyphenol interactions enhance the antioxidant capacity of phenolics: analysis of rice glutelin–procyanidin dimer interactions
Abstract
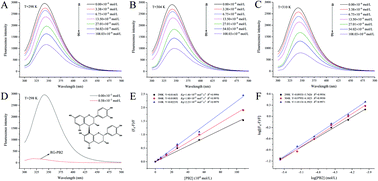
Rice glutelin and procyanidins are often used in functional foods as sources of plant-based proteins and polyphenols, respectively, but little is currently known about the interactions between them. In our research, the interaction between rice glutelin and the B-type procyanidin dimer (PB2) was investigated. The presence of the PB2 decreased the α-helix and random coil structure of the rice protein and reduced its surface hydrophobicity. However, the PB2 did not adversely affect the functional performance of RG in emulsions. Conversely, the antioxidant capacity of the PB2 was enhanced in the presence of the rice protein. Fluorescence spectroscopy confirmed that the protein and PB2 formed molecular complexes, which were primarily the result of hydrophobic attractive forces. Molecular docking analysis provides insights into the nature of the interaction between the rice protein and PB2. This study provides valuable insights into the nature of the interactions between plant proteins and polyphenolic nutraceuticals.


 Please wait while we load your content...
Please wait while we load your content...
